The Nestle baby formula recall in the UAE is a precautionary action initiated by the Emirates Drug Establishment after traces of bacteria were detected in an ingredient used in certain formulas. No illnesses have been reported in the UAE, but parents are advised to check products and avoid use if identified as part of the recall. This recall is part of wider safety measures to protect infants.
Key takeaways for UAE parents
- Selected Nestlé infant formulas are included in the recall.
- Action was taken due to the potential presence of a toxin-producing bacteria.
- Other GCC nations issued similar recalls.
- Products not listed remain safe for consumption.
What Triggered the Nestle Baby Formula Recall in the UAE?

The Nestlé baby formula recall in the UAE was initiated after a quality issue was discovered in a raw material used in the production of certain infant formulas. The Emirates Drug Establishment confirmed that this recall is voluntary and precautionary, conducted to safeguard public health.
The concern came from traces of Bacillus cereus discovered in a raw ingredient, which, under specific conditions, may result in a toxin that could cause gastrointestinal illness in infants.
The UAE recall aligns with a larger global recall, involving countries across Europe, Africa, the Americas, and Asia, where similar affected batches were distributed. While the possibility of contamination exists, no illness or adverse reactions related to these products have been reported in the UAE.
This proactive response demonstrates the country’s commitment to stringent infant safety standards and rapid action when potential risks are identified rather than waiting for cases to appear.
Which Nestlé Infant Formula Products Are Being Recalled?
Understanding which products are part of the Nestle baby formula recall is essential for parents and caregivers in the UAE. Only specific products and production batches are affected.
These products were manufactured with a raw material that may contain traces of Bacillus cereus bacteria capable of producing a toxin under certain conditions. Parents are advised to stop using the recalled items until clarification on their batch status is confirmed.
List of Affected Products in the UAE
Product Name Description
NAN Comfort 1 Infant formula for newborns
NAN Optipro 1 Standard infant formula
NAN Supreme Pro 1 Premium infant formula
NAN Supreme Pro 2 Follow-on formula
NAN Supreme Pro 3 Toddler formula
Isomil Ultima 1 Soy-based infant formula
Isomil Ultima 2 Soy follow-on formula
Isomil Ultima 3 Soy toddler formula
Alfamino Hypoallergenic formula
These items form part of the voluntary recall and are being withdrawn from store shelves, online retailers, and warehouses. It is important to note that recalls apply to batch-specific production rather than an entire product range. Only those units produced using the affected ingredient have been flagged.
Clarification of Batch-Specific Nature
Not every formula listed above is unsafe. Specific batches produced during a narrow timeframe are impacted. Parents must verify the batch number printed on the packaging to determine recall eligibility.
If your batch number does not appear on recall lists provided by trusted sources, the product is considered safe to use.
Are All Nestlé Formulas Unsafe?
No. Only certain formulas and individual batches are involved. Nestlé continues to manufacture and supply safe infant nutrition lines. All products that are not part of the recall are entirely safe when used as directed. Parents should check before discarding formula to avoid unnecessary waste.
What Is Cereulide and Why Is It Dangerous for Infants?
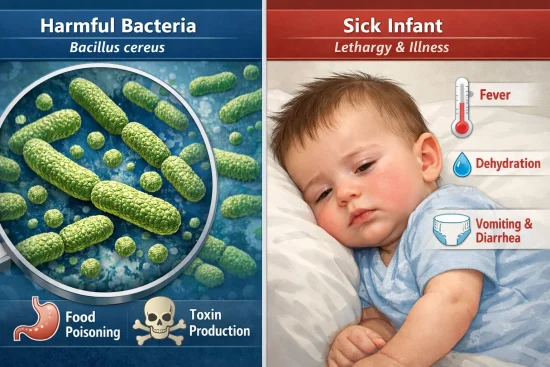
The recall relates to a potential risk posed by cereulide, a toxin that may be produced by the bacteria Bacillus cereus. Although Bacillus cereus is not unusual in the environment, it can become a concern under certain conditions when ingredients are exposed to heat or stored improperly.
Cereulide is particularly resistant to heat and may remain active even after formula is prepared correctly, which makes it different from many other common bacterial risks.
Symptoms from cereulide exposure often relate to the digestive system and can include vomiting, nausea, and stomach pain. Infants are more vulnerable because their digestive systems and immune responses are less developed compared to older children or adults.
How the Contamination Occurred?
A raw ingredient used during formula production, specifically arachidonic acid (ARA) oil, tested positive for traces of Bacillus cereus. This ingredient is normally used to support infant development and is sourced from external suppliers.
Testing identified potential contamination, prompting Nestlé to examine all formulas containing the ingredient and implement recall measures where needed. Testing and investigation are ongoing to confirm the source and ensure future batches remain safe.
Comparison of Risk Levels: Infants vs Adults
Group Risk of Cereulide Exposure
Infants Higher risk due to sensitive digestive and immune systems
Adults Lower risk due to developed immunity and stronger digestive systems
Infants are much more susceptible to toxins because they cannot tolerate the same exposures that adults can. Even a small amount can lead to uncomfortable symptoms.
Adults consuming contaminated products are less likely to experience severe illness and often recover quickly if affected. For this reason, regulators treat recalls involving baby products with extreme caution and respond quickly.
What Symptoms Should You Watch for in Your Baby?
Parents are encouraged to remain vigilant for possible symptoms that could be associated with exposure to cereulide or contaminated formula. Although illness has not been recorded in the UAE from this recall, awareness helps ensure early action.
Typical symptoms to look for include:
- Persistent or repeated vomiting
- Diarrhoea or loose stools
- Unusual lethargy or tiredness
Signs may appear within half an hour to six hours after consuming a recalled product. These symptoms can resemble stomach infections or digestive upset, so if your baby shows signs after using a formula from an affected batch, it is best to speak to a paediatrician.
Medical professionals can assess whether symptoms relate to feeding or another cause. If no symptoms occur, there is no indication of harm, yet parents should stop using recalled batches and select a safe formula until guidance confirms otherwise.
How Can You Check if You Have a Recalled Formula?

Parents can determine whether their baby formula is affected by checking the batch or lot number printed on the packaging. This is usually located on the bottom of the tin or along the side seal. Visual checks take only seconds and are the most reliable way to confirm if a product is included in the recall.
Steps to check include:
- Identify the batch number on your product
- Compare it against the recall advice provided by Nestlé or UAE authorities
- Contact helplines or customer service if uncertain
If your formula is listed, discontinue use immediately. Stores are expected to remove outdated stock, but families may still have previously purchased cans at home. Those seeking replacements or refunds should follow instructions from manufacturers or retailers.
If in doubt, err on the side of caution and temporarily switch to a confirmed safe alternative. Healthcare providers can also verify product safety when needed.
How Are UAE Authorities Ensuring Baby Product Safety?
The UAE maintains strict oversight of infant nutrition products and treats any potential risk to children seriously. Following Nestlé’s advisory, the Emirates Drug Establishment coordinated efforts across distribution channels to secure all affected batches.
This includes product quarantines in warehouses, recalls from supermarket shelves, and removal from online platforms to prevent further purchase or consumption.
Regulators follow recognised safety procedures that include verifying suppliers, tracking product batches, and collaborating with global agencies. The recall effort also involves communicating with retailers and distributors to ensure all parties follow removal guidelines.
By acting swiftly, the UAE demonstrates its strict commitment to public protection and transparency. Authorities continue to monitor imports and supply chains to prevent affected products from returning to circulation.
Parents benefit from these controls through access to updated information and the assurance that only safe, approved products remain available throughout the country.
What Is Nestlé Doing to Address the Situation?

Nestlé has responded to the recall by increasing testing, communicating openly with regulators, and withdrawing products that could pose a risk. The company performed extensive sampling of ARA oil and related mixes across all regions where production batches may have been used. Nestlé also confirmed that the issue stems from a supplier-provided ingredient and not from its own manufacturing processes.
To prevent further concern, the company is working to minimise disruptions in formula availability by expanding quality checks and offering guidance to parents and caregivers. Nestlé maintains direct contact with public health agencies to help trace and verify affected lots.
In addition to managing the recall, the company remains committed to safety, transparency, and restoring confidence in its infant nutrition portfolio. This includes ongoing root cause investigations and reinforcing supplier oversight to ensure such issues are avoided in the future.
What Should You Do if Your Baby Has Consumed a Recalled Product?
Parents whose baby has consumed a recalled formula should first stop using the product and set aside any remaining cans. Checking batch numbers allows you to confirm whether the formula is included in the recall. If the product is not on the list, it is considered safe.
Common steps include
- Noting any abnormal behaviour or symptoms
- Monitoring your baby for vomiting or stomach upset
- Contacting a paediatrician if symptoms appear
Healthcare providers can assess whether symptoms relate to the recalled formula or another cause. If your baby is healthy and has shown no symptoms despite consuming an affected batch, there is generally no need for panic.
However, feeding should switch to a safe alternative until further guidance is given. Parents should also use reliable sources for updates rather than relying on hearsay or rumours, and keep affected products for potential return or reimbursement as instructed by authorities.
What Are Other Parents in the UAE Saying?

The recall has prompted a variety of reactions among UAE parents, from concern and confusion to relief that swift action has been taken. Some parents report immediately checking tins at home and disposing of recalled products. Others are proactively advising friends, family members, and community groups to check lot numbers and switch brands temporarily if necessary.
Caregivers also express appreciation that authorities and manufacturers issued a recall before any confirmed illness cases surfaced. For many, the recall reinforces trust in the UAE’s health oversight and regulatory system. Parents active in school and neighbourhood groups share updates and help each other navigate replacement formula options.
Although anxiety is natural when dealing with infant products, many parents report feeling reassured once they learn that only a limited set of products are involved and that every effort is underway to ensure safe formula remains readily available in stores across the country.
Is This a One-Time Event or Part of a Larger Concern?
This Nestle baby formula recall appears to be a one-time event tied to a quality issue involving a supplier ingredient rather than an ongoing product safety problem.
Global recalls occasionally occur in the infant nutrition market when unexpected contamination risks are discovered. Regulations are strict and err on the side of safety to prevent illness, especially for vulnerable groups like newborns.
Nestlé’s rapid response reflects commitment to high standards and accountability rather than widespread production concerns. The affected formulas make up only a small part of Nestlé’s supply, and the company’s internal checks and investigations are designed to prevent recurrence.
Parents should remain aware of future updates, but there is no present indication of long-term supply or safety issues. With improved supplier controls and increased awareness from both parents and regulators, families can continue to rely on infant formula products as a safe source of nutrition.
Conclusion
The Nestle baby formula recall in the UAE is a responsible precaution to protect the health and wellbeing of infants. While only selected batches of specific formulas are affected, parents are urged to identify affected cans, stop using recalled products, and consult healthcare providers if concerns arise.
No illness cases have been recorded locally, yet officials have moved swiftly to secure all affected products from retail and distribution points.
Nestlé continues to investigate the supplier ingredient issue while working with regulators to safeguard future production. The Emirates Drug Establishment is monitoring the situation closely and coordinating with regional partners to ensure safe supply remains available.
By following reliable recall guidance and staying informed through official channels, UAE parents can confidently support their child’s nutrition and make decisions grounded in clarity and reassurance.
FAQs
Can I still use other Nestlé baby products?
Yes, products that are not part of the recall are considered safe. Only specific batches are affected.
How do I know if my formula has Bacillus cereus?
Only formulas with a listed batch number are included. Checking codes against official recall details confirms status.
What are the chances of my child getting sick?
No illness reports exist in the UAE linked to the recall. The recall is precautionary to avoid any risk.
Will Nestlé refund or replace the recalled product?
Refunds or replacements are generally offered for recalled items. Parents should follow provided instructions.
Can I report a reaction or issue somewhere?
Yes, parents noticing symptoms can speak to a paediatrician or contact authorities. Reports assist with monitoring.
Are formula recalls common in the UAE?
Recalls are uncommon but do occur when safety standards require them. Authorities act quickly when concerns arise.
How long will the recalled products be off the shelves?
Recalled formulas stay off shelves until fully removed and verified safe. Restocking resumes once clearance is granted.